Glioblastoma multiforme multi-omics example#
Multi-modal GBM example
In this notebook, we demonstrate the NetFlow multi-modal pipeline on TCGA multi-omic GBM data.
The data was pre-processed as described in [1], and can be found in the provided supplementary data.
Brief data description:
213 samples
mRNA gene expression (12,042 genes with 7,092 genes in largest connected component of HPRD)
DNA methylation (methy; 1,305 features)
miRNA expression (534 features)
Here, we consider mRNA gene expression for the 7,092 genes in the largest connected component of the HPRD network.
Load libraries#
from pathlib import Path
import sys
import matplotlib.pyplot as plt
import matplotlib.colors as mcolors
import networkx as nx
import numpy as np
import pandas as pd
import plotly.colors as pc
from lifelines import KaplanMeierFitter
from lifelines.plotting import add_at_risk_counts
from lifelines.statistics import logrank_test, multivariate_logrank_test
If netflow has not been installed, add the path to the library:
sys.path.insert(0, Path(Path('.').absolute()).parents[3].resolve().as_posix())
# sys.path.insert(0, Path(Path('.').absolute()).parents[0].resolve().as_posix())
import netflow as nf
import netflow.probe.clustering as nfc
import netflow.probe.visualization as nfv
Colors#
First, we set a discrete colormap with more than 20 colors for plotting later
colors = pc.qualitative.D3 + pc.qualitative.Alphabet
discrete_cmap = mcolors.ListedColormap(colors)
print(discrete_cmap.N)
discrete_cmap
36
Directories#
MAIN_DIR = Path('.').resolve() / 'example_data' / 'GBM'
DATA_DIR = MAIN_DIR / 'data'
GE_FNAME = DATA_DIR / 'gene_expression.csv'
METHY_FNAME = DATA_DIR / 'methy.csv'
MIRNA_FNAME = DATA_DIR / 'mirna.csv'
CLIN_FNAME = DATA_DIR / 'survival.csv'
RESULTS_DIR = MAIN_DIR / 'netflow_results'
if not RESULTS_DIR.is_dir():
RESULTS_DIR.mkdir()
Initialize the keeper#
keeper = nf.Keeper(outdir=RESULTS_DIR)
for dl, f in zip(['RNA', 'methy', 'miRNA', 'survival'], [GE_FNAME, METHY_FNAME, MIRNA_FNAME, CLIN_FNAME]):
keeper.load_data(f, label=dl, index_col=0)
NetFlow pipeline#
Compute distances#
We compute sample-pairwise Euclidean distances on each omic dataset:
for dd in ['RNA', 'methy', 'miRNA']:
keeper.euc_distance_pairwise_observation_profile(dd)
Compute similarities#
n_neighbors = 12
for dd in keeper.distances:
try:
keeper.compute_similarity_from_distance(dd.label, n_neighbors, 'max',
label=None, knn=False)
except Exception as e:
print(e, dd)
Fuse similarities#
keeper.similarities.keys()
dict_keys(['similarity_max12nn_RNA_profile_euc', 'similarity_max12nn_methy_profile_euc', 'similarity_max12nn_miRNA_profile_euc'])
n_neighbors = 12
# labels of similarties, as referenced in the Keeper, to fuse:
fs = [f'similarity_max{n_neighbors}nn_RNA_profile_euc',
f'similarity_max{n_neighbors}nn_methy_profile_euc',
f'similarity_max{n_neighbors}nn_miRNA_profile_euc']
# label to reference the fused similarity in the Keeper:
fsl = f"fused_similarity_max{n_neighbors}nn_RNA_methy_miRNA_euc"
# Fuse the similarities:
keeper.fuse_similarities(fs, fused_key=fsl)
Compute diffusion distances from (fused) similarities#
for sim_label in keeper.similarities.keys():
# print(sim.label)
keeper.compute_dpt_from_similarity(sim_label, density_normalize=True)
Compute the POSE#
We compute the POSE with respect to the diffusion distances for each individual modality as well as the fused multi-modal distances:
mutual = True
k_mnn = 1
min_branch_size = 10
root = 'density_inv'
root_as_tip = True
n_branches = 5
for key in keeper.distances.keys():
if not key.startswith('dpt'):
continue
g_name = f'POSE_{k_mnn}mnn_{n_branches}branches_{key}'
if g_name in keeper.graphs:
continue
poser, G_poser_nn = keeper.construct_pose(key, n_branches=n_branches,
min_branch_size=min_branch_size, # 10,
until_branched=True, verbose='ERROR',
mutual=mutual, k_mnn=k_mnn,
root=root, root_as_tip=root_as_tip)
G_poser_nn.name = g_name
# if n_branches > 3:
# if max(dict(nx.get_node_attributes(G_poser_nn,
# 'branch')).values()) == max(dict(nx.get_node_attributes(keeper.graphs[g_name.replace(f"{n_branches}branches", "3branches")],
# 'branch')).values()):
# print(f"no additional branches for {g_name}...")
# break
try:
keeper.add_graph(G_poser_nn, G_poser_nn.name)
except Exception as e:
print(f"skipping {key} - {e}...")
Probing the POSE#
BL-clustering#
for gg in keeper.graphs:
if not gg.name.startswith('POSE'):
continue
nfc.louvain_paritioned(gg, 'branch', louvain_attr='louvain0x1',
weight='inverted_distance', resolution=0.1, seed=0)
Survival analysis#
clin = keeper.data['survival'].to_frame().T
Survival analysis based on backbone branches
Kaplan-Meier Survival analysis (with logrank and multi-variate logrank p-values) shown for branches of POSEs for single-modality and fused-modality. We find that DNA methylation does have surivival information but the fused multi-omic POSE is the most descriptive in terms of survival profiles and identifies the largest lower-risk subtype.
for g_name in keeper.graphs.keys():
partition = pd.DataFrame({kk: dict(keeper.graphs[g_name].nodes.data(kk)) for kk in ['name', 'branch']}).set_index('name')['branch']
if partition.nunique() <= 10:
cmap = plt.cm.tab10
elif partition.nunique() <= 20:
cmap = plt.cm.tab20
else:
cmap = discrete_cmap
fig, ax = plt.subplots(1,1, figsize=(6.,4.2))
df = pd.concat([partition, clin], axis=1)
nfv.KM_between_groups(df['Survival'], df['Death'], df[partition.name], min_group_size=3,
ttl=g_name.split(f'max{n_neighbors}nn_')[-1].split('_euc')[0].split('_profile')[0].replace('_', ' + ') + ' POSE', # g_name.replace('from', '\nfrom').replace('max12nn', '\nmax12nn'),
precision=6,
colors=dict(zip(sorted(partition.unique()), cmap.colors)),
show_at_risk_counts=False, xlabel='time', ax=ax)
ax.set_ylabel('survival');
ax.set_ylim([0., 1]);
p_mv = multivariate_logrank_test(df['Survival'], df[partition.name], event_observed=df['Death'])
lrp = f"{p_mv.p_value:2.2e}"
if 'e' in lrp:
lrp = r"$p = {0} \times 10^{{{1}}}$".format(*lrp.split('e'))
else:
lrp = r"$p = {0}$".format(lrp)
plt.gca().text(0.45, 0.85, lrp, transform=plt.gca().transAxes, ha='left', va='bottom', fontsize='xx-large');
plt.gca().legend(fontsize='large', title=partition.name, title_fontsize='x-large', loc=(1.02, 0.))
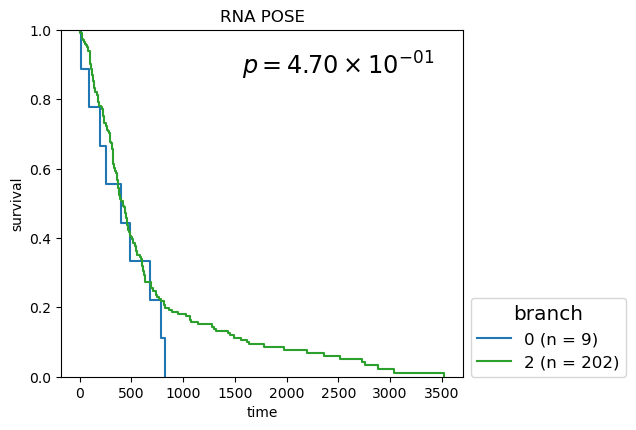
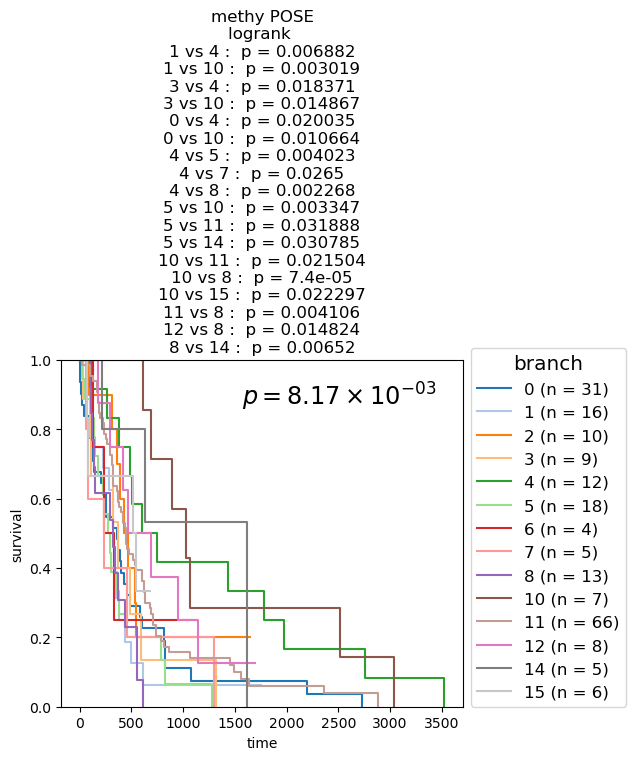
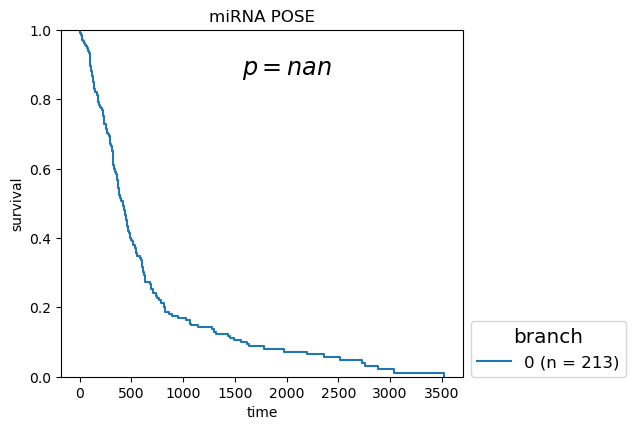
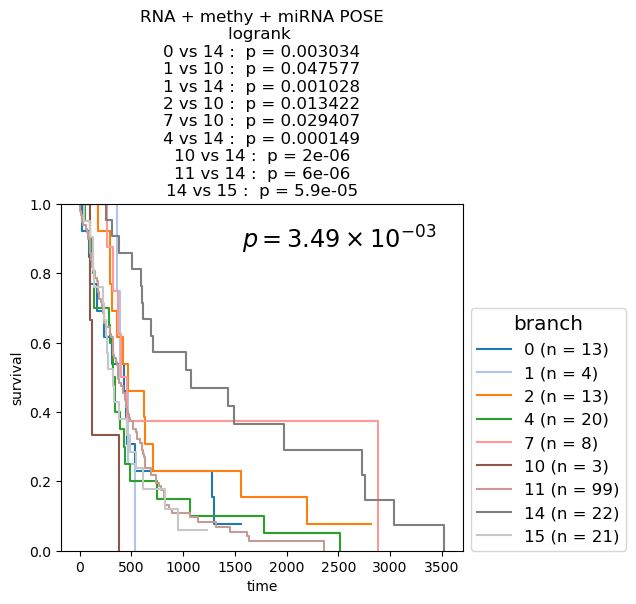
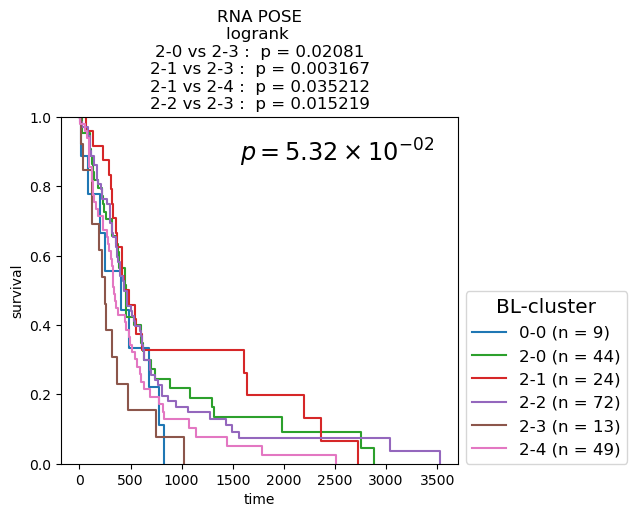
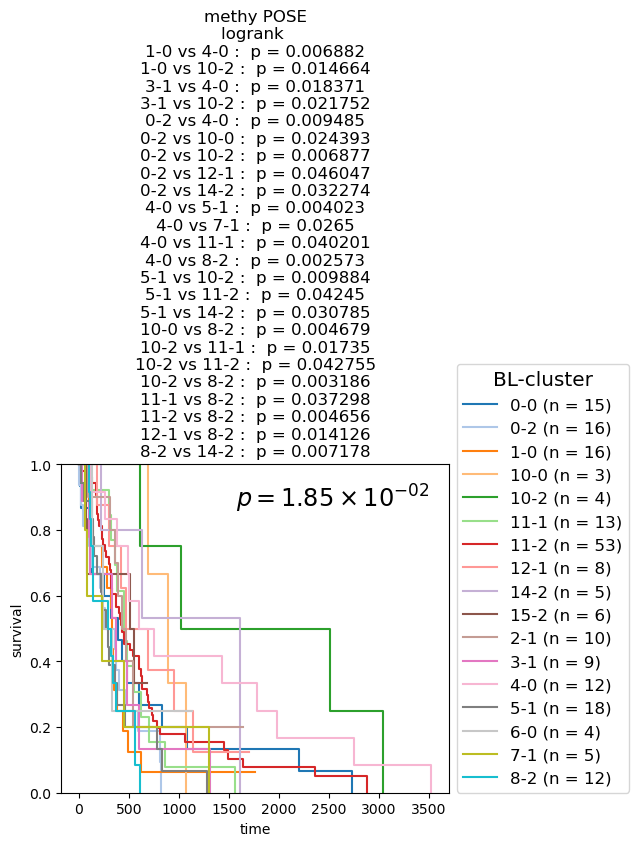
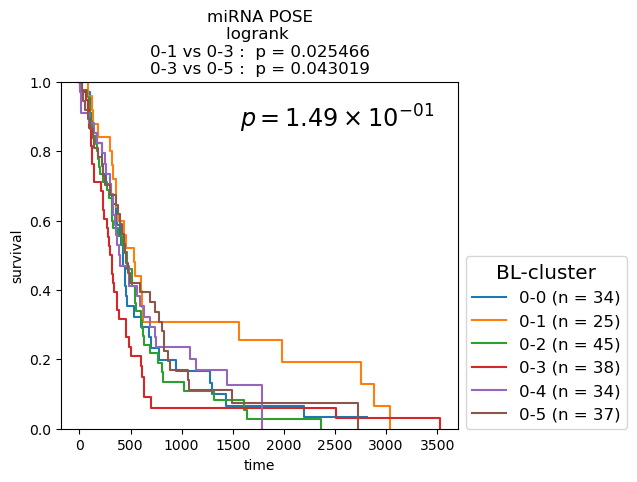
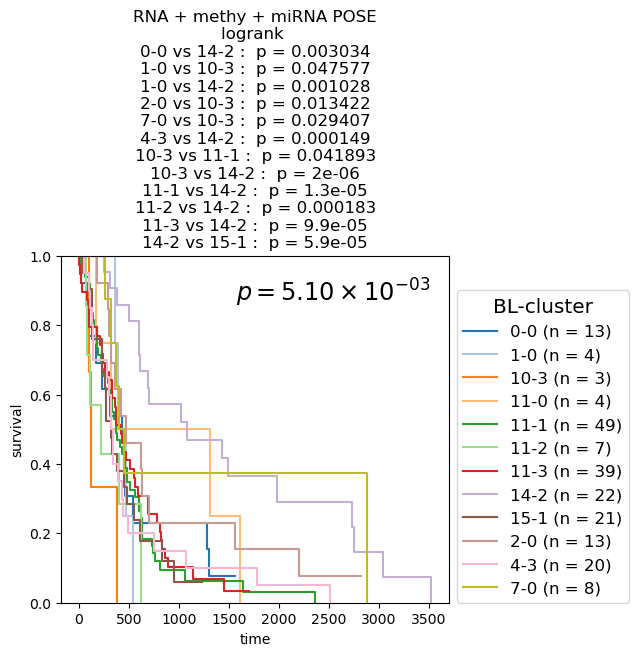
Survival analysis based on BL-clusters
Kaplan-Meier Survival analysis (with logrank and multi-variate logrank p-values) shown for BL-clusters of POSEs for single-modality and fused-modality. We find that BL-clusters provide finer resolution and more informative clustering with respect to survival. Again, the fused multi-omic POSE is the most descriptive in terms of survival profiles and identifies the largest lower-risk subtype.
for g_name in keeper.graphs.keys():
partition = pd.DataFrame({kk: dict(keeper.graphs[g_name].nodes.data(kk)) for kk in ['name', 'branch-louvain0x1']}).set_index('name')['branch-louvain0x1']
if partition.nunique() <= 10:
cmap = plt.cm.tab10
elif partition.nunique() <= 20:
cmap = plt.cm.tab20
else:
cmap = discrete_cmap
fig, ax = plt.subplots(1,1, figsize=(6.,4.2))
df = pd.concat([partition, clin], axis=1)
nfv.KM_between_groups(df['Survival'], df['Death'], df[partition.name], min_group_size=3,
ttl=g_name.split(f'max{n_neighbors}nn_')[-1].split('_euc')[0].split('_profile')[0].replace('_', ' + ') + ' POSE', # g_name.replace('from', '\nfrom').replace('max12nn', '\nmax12nn'),
precision=6,
colors=dict(zip(sorted(partition.unique()), cmap.colors)),
show_at_risk_counts=False, xlabel='time', ax=ax)
ax.set_ylabel('survival');
ax.set_ylim([0., 1]);
p_mv = multivariate_logrank_test(df['Survival'], df[partition.name], event_observed=df['Death'])
lrp = f"{p_mv.p_value:2.2e}"
if 'e' in lrp:
lrp = r"$p = {0} \times 10^{{{1}}}$".format(*lrp.split('e'))
else:
lrp = r"$p = {0}$".format(lrp)
plt.gca().text(0.45, 0.85, lrp, transform=plt.gca().transAxes, ha='left', va='bottom', fontsize='xx-large');
plt.gca().legend(fontsize='large', title='BL-cluster', title_fontsize='x-large', loc=(1.02, 0.))
Interactive visualization#
key = 'dpt_from_transitions_sym_fused_similarity_max12nn_RNA_methy_miRNA_euc_density_normalized'
pose_key = f"POSE_1mnn_5branches_{key}"
nf.render_pose(keeper, pose_key, key, port=1782)
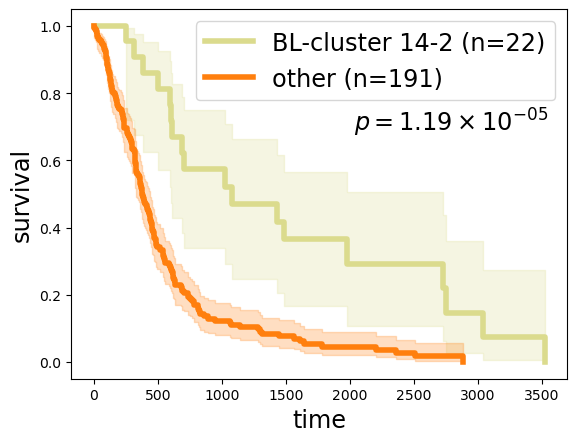
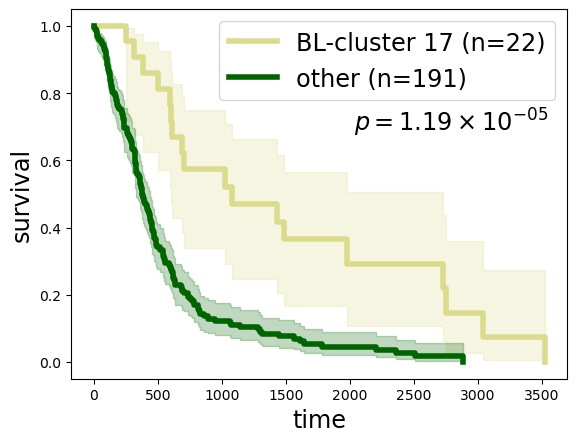
Survival analysis between BL-cluster 14-2 (or equivalently, branch 14) and the rest of the cohort, reveals that BL-cluster 14-2 is a lower-risk GBM subtype:
ll = 'TCGA-02-0084, TCGA-06-0146, TCGA-06-0221, TCGA-02-0258, TCGA-02-0116, TCGA-02-0069, TCGA-02-0104, TCGA-02-0080, TCGA-02-0010, TCGA-06-0129, TCGA-02-0114, TCGA-02-0074, TCGA-06-0128, TCGA-02-0058, TCGA-02-0432, TCGA-08-0344, TCGA-02-0087, TCGA-06-0686, TCGA-08-0516, TCGA-02-0007, TCGA-02-0028, TCGA-06-0178'
ll = ll.split(', ')
rr = list(set(clin.index) - set(ll))
print(len(ll))
print(len(rr))
fig, ax = plt.subplots(1, 1)
kmf1 = KaplanMeierFitter()
kmf2 = KaplanMeierFitter()
kmf1.fit(clin.loc[ll, 'Survival'], event_observed=clin.loc[ll, 'Death'], label=f'BL-cluster 14-2 (n={len(ll)})')
kmf2.fit(clin.loc[rr, 'Survival'], event_observed=clin.loc[rr, 'Death'], label=f'other (n={len(rr)})')
lr = logrank_test(clin.loc[ll, 'Survival'], clin.loc[rr, 'Survival'], event_observed_A=clin.loc[ll, 'Death'], event_observed_B=clin.loc[rr, 'Death'])
# kmf1.plot(ax=ax, lw=4, color=plt.cm.tab20.colors[14])
kmf1.plot(ax=ax, lw=4, color=plt.cm.tab20.colors[17])
kmf2.plot(ax=ax, lw=4)
ax.set_xlabel('time', fontsize='xx-large');
ax.set_ylabel('survival', fontsize='xx-large');
ax.legend(loc='upper right', fontsize='xx-large');
lrp = f"{lr.p_value:2.2e}"
if 'e' in lrp:
lrp = r"$p = {0} \times 10^{{{1}}}$".format(*lrp.split('e'))
else:
lrp = r"$p = {0}$".format(lrp)
ax.text(0.57, 0.65, lrp, transform=ax.transAxes, ha='left', va='bottom', fontsize='xx-large');
22
191
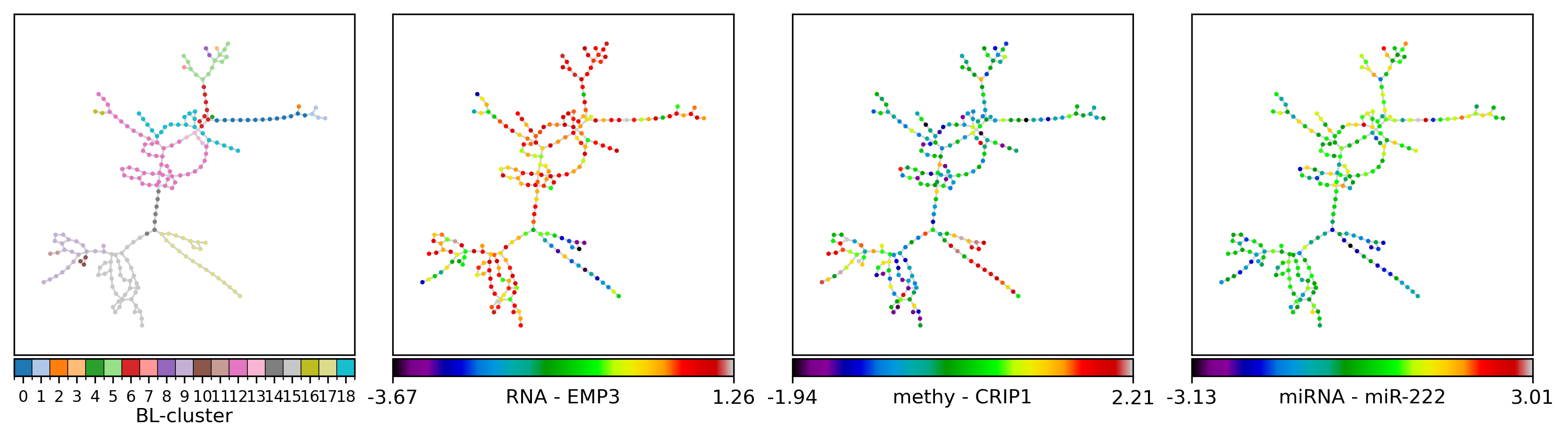
Panel visualization#
To further investigate the fused POSE, we can look at side-by-side panels of the POSE color-coded by its clustering:
s = None
gls = {'RNA': ['EMP3', # 'HSPA6',
# 'TIMP1', # 'EFEMP2', 'FLJ11286', # 'PLA2G2A', 'MAPK8', 'ADM', 'CSRP1', 'KCNE4',
],
'methy': ['CRIP1_P274_F', # 'FES_P223_R', # 'IL17RB_E164_R', 'RAB32_E314_R', 'PAX6_P1121_F', # 'PYCARD_E87_F', 'HDAC1_P414_R', 'FRZB_P406_F', 'ISL1_P379_F', 'NR2F6_E375_R',
],
'miRNA': ['hsa-miR-222', # 'hsa-miR-221', # 'hsa-miR-34a', 'hsa-miR-34b', 'hsa-miR-328', # 'hsa-miR-340', 'hsa-miR-17-3p', 'hsa-miR-197', 'hsa-miR-181d', 'hsa-miR-155',
],
}
key = 'dpt_from_transitions_sym_fused_similarity_max12nn_RNA_methy_miRNA_euc_density_normalized'
g_name = f'POSE_1mnn_5branches_{key}'
G_poser_nn = keeper.graphs[g_name]
pos = nx.layout.kamada_kawai_layout(G_poser_nn)
nl = list(G_poser_nn)
if s is None:
border_linewidths = 0
else:
s_names = {G_poser_nn.nodes[k]['name']: k for k in G_poser_nn}
s = set([s_names[k] for k in s])
border_linewidths = [0.6 if k in s else 0. for k in nl]
bordercolors = 'k'
bl = pd.Series(dict(nx.get_node_attributes(G_poser_nn, 'branch-louvain0x1')))
bl_code = dict(zip(bl.unique(), range(bl.nunique())))
record_colors = {# 'branch': [G_poser_nn.nodes[k]['branch'] for k in nl],
'BL-cluster': [bl_code[G_poser_nn.nodes[k]['branch-louvain0x1']] for k in nl],
}
# clin_rr = clin.rename(index={G_poser_nn.nodes[k]['name']: k for k in G_poser_nn}, columns={'Survival': 'OS', 'Death': 'OS status'})
# clin_rr = {k: vl.loc[nl].values for k, vl in clin_rr.iteritems()}
rr = pd.concat([keeper.data[r].to_frame().loc[gl].T.rename(columns={k: ' - '.join([r, k]) for k in gl}) for r, gl in gls.items()], axis=1)
rr = rr.rename(index={G_poser_nn.nodes[k]['name']: k for k in G_poser_nn})
rr = {k: vl.loc[nl].values for k, vl in rr.iteritems()}
record_colors = {**record_colors, **rr} # {**record_colors, **clin_rr, **rr}
bl_ntm = {ss: s for s, ss in bl_code.items()}
fig, axes = plt.subplots(1, 4, figsize=(11, 3), constrained_layout=True, dpi=300)
for (lbl, nc), ax in zip(record_colors.items(), np.ravel(axes)):
if lbl=='OS status':
ntm = {0: 'alive', 1: 'deceased'}
c = 'tab10'
node_cbar_ticks_kws = None
elif lbl in ['branch', 'Louvain (0.1)']:
if len(set(nc)) > 25:
ntm = {kk: str(kk) if kk in [min(nc), max(nc)] else '' for kk in set(nc)}
node_cbar_ticks_kws = None
else:
ntm = None
node_cbar_ticks_kws = {'fontsize': 8}
c = 'tab10' if len(set(nc)) <= 10 else 'tab20' if len(set(nc)) <= 20 else discrete_cmap
elif lbl == 'BL-cluster':
if len(set(nc)) > 25: # 15:
ntm = {kk: str(kk) if kk in [min(nc), max(nc)] else '' for kk in set(nc)} # {kk: bl_ntm[kk] if kk in [min(nc), max(nc)] else '' for kk in set(nc)}
node_cbar_ticks_kws = None
else:
ntm = None # bl_ntm
node_cbar_ticks_kws = {'fontsize': 8} # , 'rotation': 90}
c = 'tab10' if len(set(nc)) <= 10 else 'tab20' if len(set(nc)) <= 20 else discrete_cmap
else:
ntm = None
c = 'nipy_spectral'
node_cbar_ticks_kws = None # {'fontsize': 8} # None
nfv.plot_topology(G_poser_nn, pos=pos, nodelist=nl, ax=ax,
node_color=nc, node_shape='o', # ns,
node_cmap=c, node_cbar=True, node_size=5,
edge_color='lightgray', node_cbar_label=lbl.replace('_P274_F', '').replace('hsa-', ''),
node_ticklabels_mapper=ntm, node_cbar_ticks_kws=node_cbar_ticks_kws,
border_linewidths=border_linewidths, bordercolors=bordercolors)
# fig.savefig('GBM_pose.png', dpi=300, bbox_inches='tight', transparent=True)
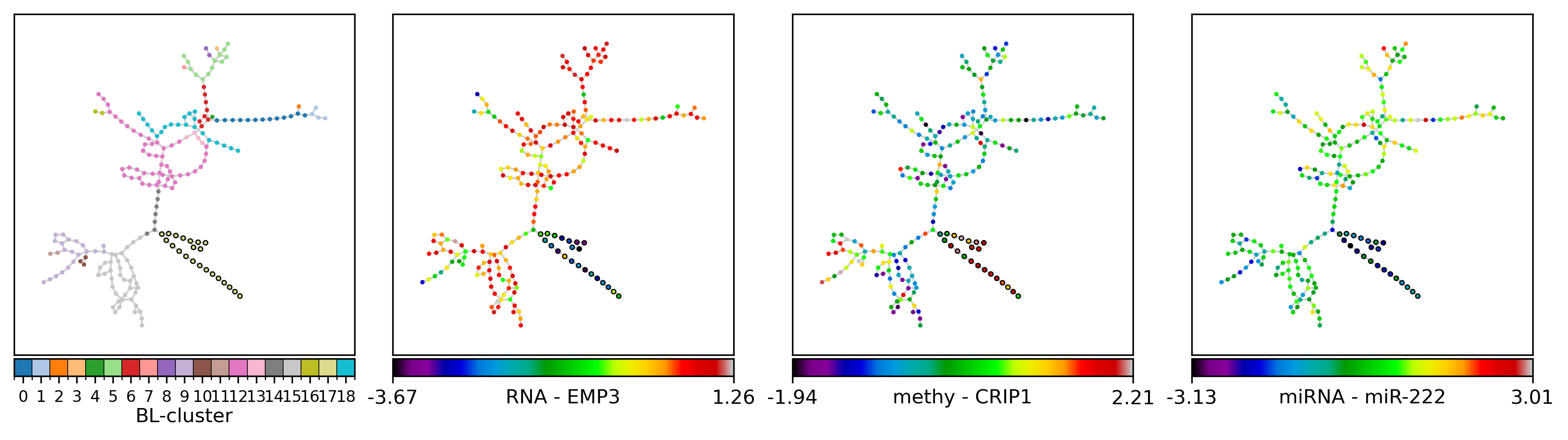
And we can create the same figure, annotating the selected samples as follows:
s = 'TCGA-02-0084, TCGA-06-0146, TCGA-06-0221, TCGA-02-0258, TCGA-02-0116, TCGA-02-0069, TCGA-02-0104, TCGA-02-0080, TCGA-02-0010, TCGA-06-0129, TCGA-02-0114, TCGA-02-0074, TCGA-06-0128, TCGA-02-0058, TCGA-02-0432, TCGA-08-0344, TCGA-02-0087, TCGA-06-0686, TCGA-08-0516, TCGA-02-0007, TCGA-02-0028, TCGA-06-0178'
s = s.split(', ')
gls = {'RNA': ['EMP3', # 'HSPA6',
# 'TIMP1', # 'EFEMP2', 'FLJ11286', # 'PLA2G2A', 'MAPK8', 'ADM', 'CSRP1', 'KCNE4',
],
'methy': ['CRIP1_P274_F', # 'FES_P223_R', # 'IL17RB_E164_R', 'RAB32_E314_R', 'PAX6_P1121_F', # 'PYCARD_E87_F', 'HDAC1_P414_R', 'FRZB_P406_F', 'ISL1_P379_F', 'NR2F6_E375_R',
],
'miRNA': ['hsa-miR-222', # 'hsa-miR-221', # 'hsa-miR-34a', 'hsa-miR-34b', 'hsa-miR-328', # 'hsa-miR-340', 'hsa-miR-17-3p', 'hsa-miR-197', 'hsa-miR-181d', 'hsa-miR-155',
],
}
key = 'dpt_from_transitions_sym_fused_similarity_max12nn_RNA_methy_miRNA_euc_density_normalized'
g_name = f'POSE_1mnn_5branches_{key}'
G_poser_nn = keeper.graphs[g_name]
pos = nx.layout.kamada_kawai_layout(G_poser_nn)
nl = list(G_poser_nn)
if s is None:
border_linewidths = 0
else:
s_names = {G_poser_nn.nodes[k]['name']: k for k in G_poser_nn}
s = set([s_names[k] for k in s])
border_linewidths = [0.6 if k in s else 0. for k in nl]
bordercolors = 'k'
bl = pd.Series(dict(nx.get_node_attributes(G_poser_nn, 'branch-louvain0x1')))
bl_code = dict(zip(bl.unique(), range(bl.nunique())))
record_colors = {# 'branch': [G_poser_nn.nodes[k]['branch'] for k in nl],
'BL-cluster': [bl_code[G_poser_nn.nodes[k]['branch-louvain0x1']] for k in nl],
}
# clin_rr = clin.rename(index={G_poser_nn.nodes[k]['name']: k for k in G_poser_nn}, columns={'Survival': 'OS', 'Death': 'OS status'})
# clin_rr = {k: vl.loc[nl].values for k, vl in clin_rr.iteritems()}
rr = pd.concat([keeper.data[r].to_frame().loc[gl].T.rename(columns={k: ' - '.join([r, k]) for k in gl}) for r, gl in gls.items()], axis=1)
rr = rr.rename(index={G_poser_nn.nodes[k]['name']: k for k in G_poser_nn})
rr = {k: vl.loc[nl].values for k, vl in rr.iteritems()}
record_colors = {**record_colors, **rr} # {**record_colors, **clin_rr, **rr}
bl_ntm = {ss: s for s, ss in bl_code.items()}
fig, axes = plt.subplots(1, 4, figsize=(11, 3), constrained_layout=True, dpi=300)
for (lbl, nc), ax in zip(record_colors.items(), np.ravel(axes)):
if lbl=='OS status':
ntm = {0: 'alive', 1: 'deceased'}
c = 'tab10'
node_cbar_ticks_kws = None
elif lbl in ['branch', 'Louvain (0.1)']:
if len(set(nc)) > 25:
ntm = {kk: str(kk) if kk in [min(nc), max(nc)] else '' for kk in set(nc)}
node_cbar_ticks_kws = None
else:
ntm = None
node_cbar_ticks_kws = {'fontsize': 8}
c = 'tab10' if len(set(nc)) <= 10 else 'tab20' if len(set(nc)) <= 20 else discrete_cmap
elif lbl == 'BL-cluster':
if len(set(nc)) > 25: # 15:
ntm = {kk: str(kk) if kk in [min(nc), max(nc)] else '' for kk in set(nc)} # {kk: bl_ntm[kk] if kk in [min(nc), max(nc)] else '' for kk in set(nc)}
node_cbar_ticks_kws = None
else:
ntm = None # bl_ntm
node_cbar_ticks_kws = {'fontsize': 8} # , 'rotation': 90}
c = 'tab10' if len(set(nc)) <= 10 else 'tab20' if len(set(nc)) <= 20 else discrete_cmap
else:
ntm = None
c = 'nipy_spectral'
node_cbar_ticks_kws = None # {'fontsize': 8} # None
nfv.plot_topology(G_poser_nn, pos=pos, nodelist=nl, ax=ax,
node_color=nc, node_shape='o', # ns,
node_cmap=c, node_cbar=True, node_size=5,
edge_color='lightgray', node_cbar_label=lbl.replace('_P274_F', '').replace('hsa-', ''),
node_ticklabels_mapper=ntm, node_cbar_ticks_kws=node_cbar_ticks_kws,
border_linewidths=border_linewidths, bordercolors=bordercolors)
# fig.savefig('GBM_pose_selected.png', dpi=300, bbox_inches='tight', transparent=True)
as well as characteristic features significantly associated with the identifed lower-risk subtype:
ll = 'TCGA-02-0084, TCGA-06-0146, TCGA-06-0221, TCGA-02-0258, TCGA-02-0116, TCGA-02-0069, TCGA-02-0104, TCGA-02-0080, TCGA-02-0010, TCGA-06-0129, TCGA-02-0114, TCGA-02-0074, TCGA-06-0128, TCGA-02-0058, TCGA-02-0432, TCGA-08-0344, TCGA-02-0087, TCGA-06-0686, TCGA-08-0516, TCGA-02-0007, TCGA-02-0028, TCGA-06-0178'
ll = ll.split(', ')
bl_mapped = bl.map(bl_code)
bl_mapped = bl_mapped.rename(index=dict(nx.get_node_attributes(G_poser_nn, 'name')))
# display(bl_mapped.value_counts())
bl_mapped.loc[ll]
TCGA-02-0084 17
TCGA-06-0146 17
TCGA-06-0221 17
TCGA-02-0258 17
TCGA-02-0116 17
TCGA-02-0069 17
TCGA-02-0104 17
TCGA-02-0080 17
TCGA-02-0010 17
TCGA-06-0129 17
TCGA-02-0114 17
TCGA-02-0074 17
TCGA-06-0128 17
TCGA-02-0058 17
TCGA-02-0432 17
TCGA-08-0344 17
TCGA-02-0087 17
TCGA-06-0686 17
TCGA-08-0516 17
TCGA-02-0007 17
TCGA-02-0028 17
TCGA-06-0178 17
dtype: int64
ll = 'TCGA-02-0084, TCGA-06-0146, TCGA-06-0221, TCGA-02-0258, TCGA-02-0116, TCGA-02-0069, TCGA-02-0104, TCGA-02-0080, TCGA-02-0010, TCGA-06-0129, TCGA-02-0114, TCGA-02-0074, TCGA-06-0128, TCGA-02-0058, TCGA-02-0432, TCGA-08-0344, TCGA-02-0087, TCGA-06-0686, TCGA-08-0516, TCGA-02-0007, TCGA-02-0028, TCGA-06-0178'
ll = ll.split(', ')
rr = list(set(clin.index) - set(ll))
print(len(ll))
print(len(rr))
fig, ax = plt.subplots(1, 1)
kmf1 = KaplanMeierFitter()
kmf2 = KaplanMeierFitter()
# kmf1.fit(clin.loc[ll, 'Survival'], event_observed=clin.loc[ll, 'Death'], label=f'BL-cluster 14-2 (n={len(ll)})')
kmf1.fit(clin.loc[ll, 'Survival'], event_observed=clin.loc[ll, 'Death'], label=f'BL-cluster 17 (n={len(ll)})')
kmf2.fit(clin.loc[rr, 'Survival'], event_observed=clin.loc[rr, 'Death'], label=f'other (n={len(rr)})')
lr = logrank_test(clin.loc[ll, 'Survival'], clin.loc[rr, 'Survival'], event_observed_A=clin.loc[ll, 'Death'], event_observed_B=clin.loc[rr, 'Death'])
# kmf1.plot(ax=ax, lw=4, color=plt.cm.tab20.colors[14])
kmf1.plot(ax=ax, lw=4, color=plt.cm.tab20.colors[17])
kmf2.plot(ax=ax, lw=4, color='darkgreen')
ax.set_xlabel('time', fontsize='xx-large');
ax.set_ylabel('survival', fontsize='xx-large');
ax.legend(loc='upper right', fontsize='xx-large');
lrp = f"{lr.p_value:2.2e}"
if 'e' in lrp:
lrp = r"$p = {0} \times 10^{{{1}}}$".format(*lrp.split('e'))
else:
lrp = r"$p = {0}$".format(lrp)
ax.text(0.57, 0.65, lrp, transform=ax.transAxes, ha='left', va='bottom', fontsize='xx-large');
# fig.savefig('GBM_BLcluster17_KM.png', dpi=300, bbox_inches='tight', transparent=True)
22
191
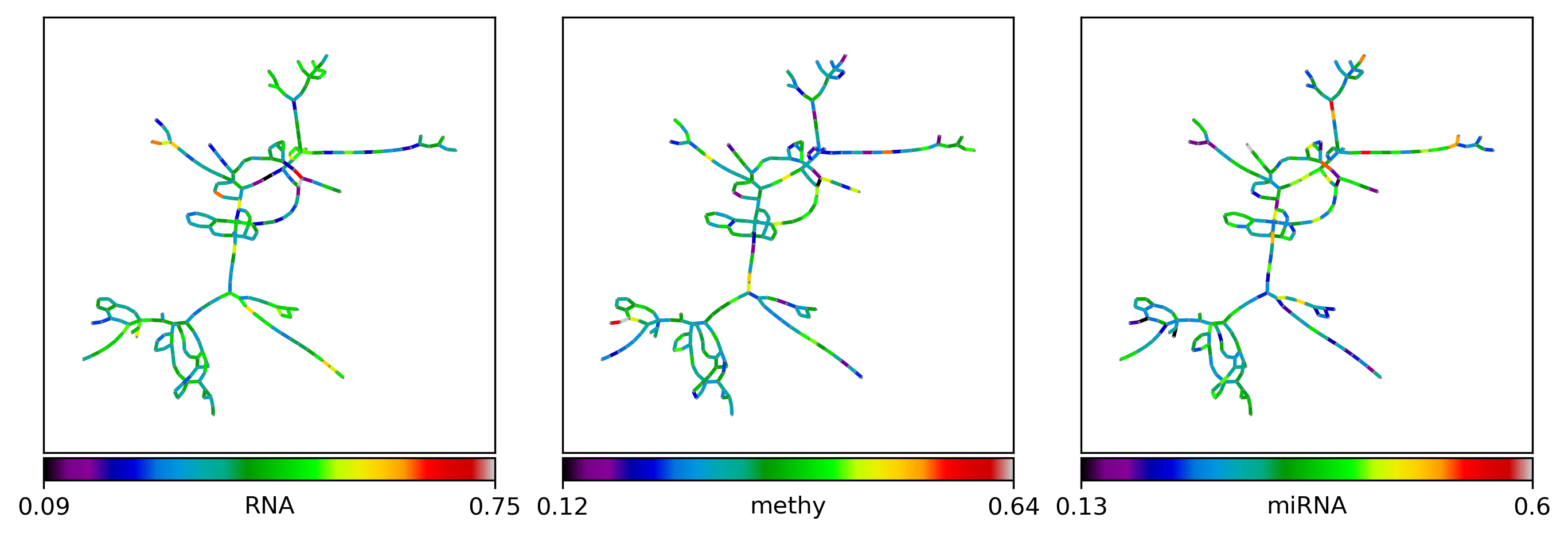
Inspect modality proportion of influence#
refs = {'RNA': 'transitions_sym_similarity_max12nn_RNA_profile_euc_density_normalized',
'methy': 'transitions_sym_similarity_max12nn_methy_profile_euc_density_normalized',
'miRNA': 'transitions_sym_similarity_max12nn_miRNA_profile_euc_density_normalized',
'fused': 'transitions_sym_fused_similarity_max12nn_RNA_methy_miRNA_euc_density_normalized',
}
edge_attr = {v: {modality: keeper.misc[P][v] for modality, P in refs.items()} for v in G_poser_nn.edges()}
edge_attr_proportions = {}
for v, attr in edge_attr.items():
mn = sum([attr[k] for k in ['RNA', 'methy', 'miRNA']])
edge_attr_proportions[v] = {'_'.join([k, 'proportion']) : attr[k] / mn for k in ['RNA', 'methy', 'miRNA']}
nx.set_edge_attributes(G_poser_nn, edge_attr_proportions)
visualization#
s = None
gls = {'RNA': ['EMP3', # 'HSPA6',
# 'TIMP1', # 'EFEMP2', 'FLJ11286', # 'PLA2G2A', 'MAPK8', 'ADM', 'CSRP1', 'KCNE4',
],
'methy': ['CRIP1_P274_F', # 'FES_P223_R', # 'IL17RB_E164_R', 'RAB32_E314_R', 'PAX6_P1121_F', # 'PYCARD_E87_F', 'HDAC1_P414_R', 'FRZB_P406_F', 'ISL1_P379_F', 'NR2F6_E375_R',
],
'miRNA': ['hsa-miR-222', # 'hsa-miR-221', # 'hsa-miR-34a', 'hsa-miR-34b', 'hsa-miR-328', # 'hsa-miR-340', 'hsa-miR-17-3p', 'hsa-miR-197', 'hsa-miR-181d', 'hsa-miR-155',
],
}
key = 'dpt_from_transitions_sym_fused_similarity_max12nn_RNA_methy_miRNA_euc_density_normalized'
g_name = f'POSE_1mnn_5branches_{key}'
G_poser_nn = keeper.graphs[g_name]
pos = nx.layout.kamada_kawai_layout(G_poser_nn)
nl = list(G_poser_nn)
el = list(G_poser_nn.edges())
ee = pd.DataFrame({k: dict(nx.get_edge_attributes(G_poser_nn, '_'.join([k, 'proportion']))) for k in ['RNA', 'methy', 'miRNA']})
ee = ee.loc[el]
if s is None:
border_linewidths = 0
else:
s_names = {G_poser_nn.nodes[k]['name']: k for k in G_poser_nn}
s = set([s_names[k] for k in s])
border_linewidths = [0.6 if k in s else 0. for k in nl]
bordercolors = 'k'
fig, axes = plt.subplots(1, 3, figsize=(9, 3), constrained_layout=True, dpi=300)
ntm = None
c = plt.cm.nipy_spectral
node_cbar_ticks_kws = None
for (lbl, ec), ax in zip(ee.iteritems(), np.ravel(axes)):
nfv.plot_topology(G_poser_nn, pos=pos, nodelist=nl, ax=ax,
node_color='gray', node_shape='o', # ns,
edge_cmap=c, edge_cbar=True, node_size=1,
edge_width=1.5,
edge_color=ec.values, edge_cbar_label=lbl,
# node_ticklabels_mapper=ntm, node_cbar_ticks_kws=node_cbar_ticks_kws,
border_linewidths=border_linewidths, bordercolors=bordercolors,
edge_cbar_kws={'location': 'bottom', 'orientation': 'horizontal', 'pad': 0.01}
)
# fig.savefig('Downloads/GBM_pose_edges.png',
# dpi=300, bbox_inches='tight', transparent=True)
References#
[1] Wang, B., Mezlini, A.M., Demir, F., Fiume, M., Tu, Z., Brudno, M., Haibe-Kains, B. and Goldenberg, A., 2014. Similarity network fusion for aggregating data types on a genomic scale. Nature methods, 11(3), pp.333-337. https://www.nature.com/articles/nmeth.2810